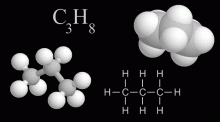
 Propane is a hydrocarbon based fuel with the moleclar formula C3H8, normally a gas but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used for residental heating and cooking, as a fuel for engines, generators, torches, barbecues, and portable stoves.
Propane is a hydrocarbon based fuel with the moleclar formula C3H8, normally a gas but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used for residental heating and cooking, as a fuel for engines, generators, torches, barbecues, and portable stoves.
Propane in its natural state is colurless and ordourless, for safety reasons propane is required to be odorized as a method of detection, a scent similar to rotten eggs or boiling cabbage. Propane is stored under pressure as a liquid with a boiling tempurature of -42ºC, at which point it vaporizes from propane liquid to propane vapour. Propane is bought and stored in a liquid form (LPG). With proper combustion, propane produces about 50MJ/kg. Propane combustion is much cleaner than gasoline combustion. About 23.5 cubic feet of air is required to burn one cubic foot of propane.
Liquified propane expands to vapour propane at a rate of one unit of liquified propane to 270 units of vapour propane. Unlike natural gas, propane is heavier than air (1.5 times as dense). In its raw state propane sinks and pools at the floor. Liquid propane will flash to a vapour at atmospheric pressure and appears white due to moisture condensing from the air. Propane is bought and stored in a liquid form (LPG).


